Evidence for the benefit of early treatment in MS – what’s new?
Reviewed by Gavin Giovannoni, Professor of Neurology, Blizard Institute, Barts and The London School of Medicine and Dentistry, UK, and Chair of MS Brain Health Steering Committee
The policy report Brain health: time matters in multiple sclerosis, launched in 2015, presented the evidence base for a more urgent approach to the management of MS. It called for timely intervention with therapies most likely to provide optimal benefit and safety for each person with MS on an individualized basis.
Since then, new treatments have become available, and more evidence is emerging for their efficacy in reducing relapse rates and slowing disability and disease progression. The tables below summarize the key findings from several important MS registries, studies and review papers published between 2016 and 2021.
Findings from registries
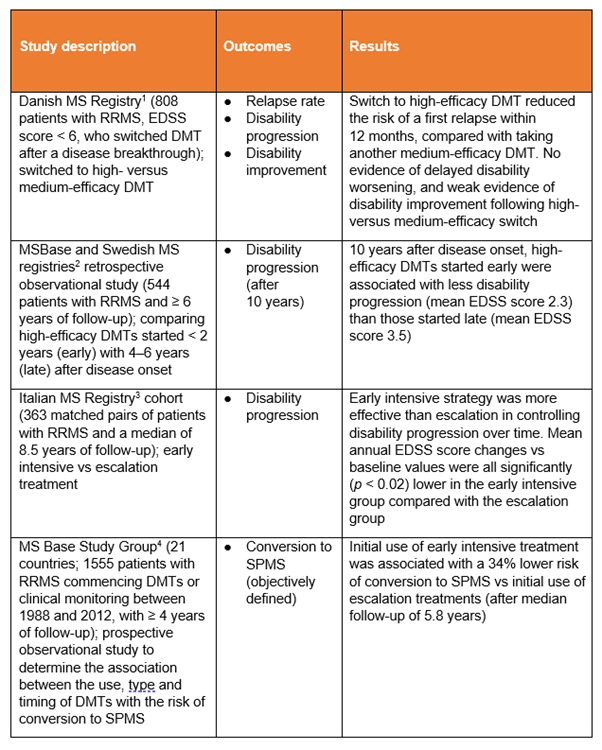
Medium-efficacy, or escalation, therapies used in these studies include azathioprine, dimethyl fumarate, glatiramer acetate, interferons and teriflunomide (not all studies assessed the same drugs).
High-efficacy or early intensive therapies used in these studies include alemtuzumab, cladribine, fingolimod, mitoxantrone, natalizumab, ocrelizumab and rituximab (not all studies assessed the same drugs).
DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; RRMS, relapsing-remitting MS; SPMS, secondary progressive MS
Some interesting trends emerge from these registry findings.
- Switching from a medium- to a high-efficacy disease-modifying therapy (DMT) reduced the risk of a further early relapse, compared with switching to another medium-efficacy DMT.1
- High-efficacy treatment initiated soon after disease onset resulted in less disability progression at 10 years than delaying high-efficacy treatment for 4–6 years.2
- Early intensive treatment was more effective than treatment escalation in controlling disability progression (over a median of 8.5 years).3
- Early intensive treatment was associated with a lower risk of conversion from relapsing-remitting MS (RRMS) to secondary progressive MS compared with escalation treatment (after a median follow-up of 5.8 years).4
Findings from published trials and real-life settings
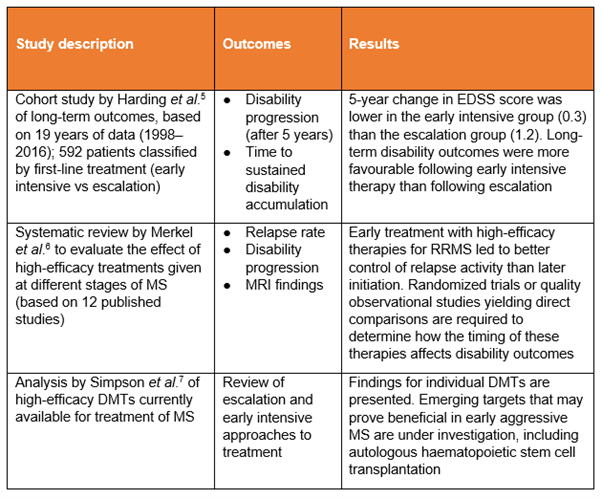
Medium-efficacy, or escalation, therapies used in these studies include azathioprine, dimethyl fumarate, glatiramer acetate, interferons and teriflunomide (not all studies assessed the same drugs).
High-efficacy or early intensive therapies used in these studies include alemtuzumab, cladribine, fingolimod, mitoxantrone, natalizumab, ocrelizumab and rituximab (not all studies assessed the same drugs).
DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; MRI, magnetic resonance imaging; RRMS, relapsing-remitting MS
These publications provide further evidence of the benefits of early intervention, with the review papers6,7 presenting a valuable overview from more studies than we can document here.
- Long-term outcomes were more favourable following early intensive therapy than with first-line moderate-efficacy DMTs.5
- Early treatment with high-efficacy therapies for RRMS led to better control of relapse activity than later initiation. Direct evidence was limited, however, so this assessment was derived mainly from extension studies and subgroup analyses (that is, analysis of findings from subsets of participants with defined characteristics within randomized clinical trials).6
- Direct comparisons are required to determine how the timing of the therapies assessed affects disability outcomes.6
There are, therefore, accumulating data for a therapeutic window of opportunity early on in MS to maximize long-term therapeutic benefits.8
Relevance of MS Brain Health consensus standards
The MS Brain Health initiative emphasizes the importance of timely intervention, where appropriate, with effective treatments that modify the disease course. This is reflected in the recommendations of the Time matters report9 and many of the treatment-related consensus standards10 derived from it.
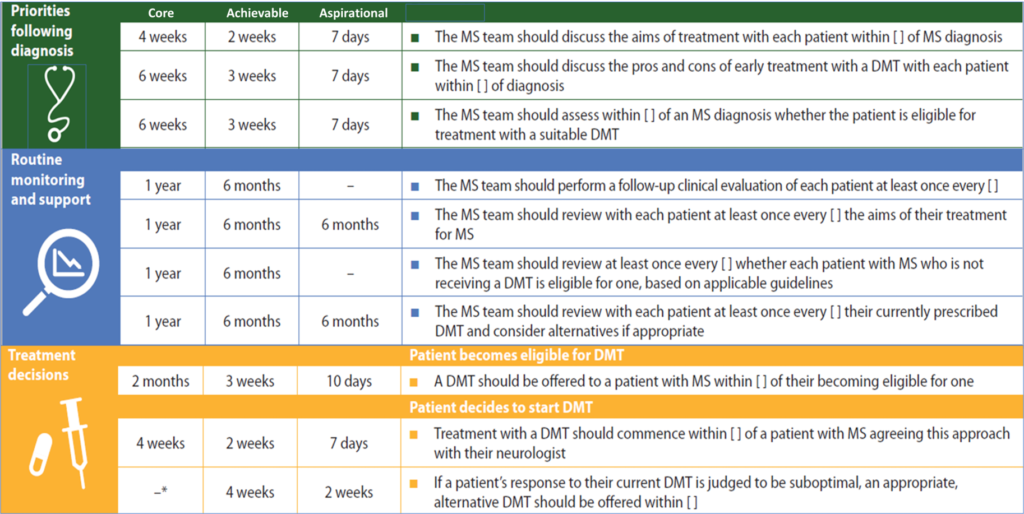
MS Brain Health consensus standards relating to early intervention with DMTs.10
*Time frame of 3 months was agreed by the experts after completion of the Delphi process.
DMT, disease-modifying therapy.
Further considerations
Despite the growing body of evidence from MS registries, studies and review papers, there is diversity of opinion among the MS community about whether to initiate treatment with ‘established’ (medium-efficacy) DMTs or with newer (high-efficacy) DMTs, and how best to approach switching therapy in the case of a suboptimal response. Among the important considerations are:
- the management of women with MS before, during and after pregnancy (see our evidence summary from the MSBase Pregnancy Research Group)
- the risk of infection and other complications associated with immune therapies.
A nationwide register-based study assessed the records of 6421 Swedish patients with RRMS receiving high-efficacy DMTs against a cohort of 42 645 age- and sex-matched individuals from the general population.11 The researchers concluded that patients with MS are at increased risk of infections, and this differs by treatment. This highlights the importance of risk-benefit assessments to inform decisions about personalized care.
Large-scale studies in progress
The Patient-Centered Outcomes Research Institute (PCORI) has funded two ongoing studies: DELIVER-MS12 and TREAT-MS.13 Patients with RRMS are being randomized to receive escalation or high-efficacy DMT. The primary outcomes are brain volume loss at 3 years and time to sustained clinical disability up to 4.5 years.14 It may be some time before the final results emerge, but these PCORI-funded studies and other ongoing research should provide a firm basis from which people with MS and their MS team can choose the best treatment approach for them, to improve their quality of life.15
References
1. Chalmer TA, Kalincik T, Laursen B et al. Treatment escalation leads to fewer relapses compared with switching to another moderately effective therapy. J Neurol 2019;266:306-15.
2. He A, Merkel B, Brown JWL et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol 2020;19:307-16.
3. Iaffaldano P, Lucisano G, Caputo F et al. Long-term disability trajectories in relapsing multiple sclerosis patients treated with early intensive or escalation treatment strategies. Ther Adv Neurol Disord 2021;14:1‒10.
4. Brown JWL, Coles A, Horakova D et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA 2019;321:175-87.
5. Harding K, Williams O, Willis M et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol 2019;76:536-41.
6. Merkel B, Butzkueven H, Traboulsee AL et al. Timing of high-efficacy therapy in relapsing-remitting multiple sclerosis: A systematic review. Autoimmun Rev 2017;16:658-65.
7. Simpson A, Mowry EM, Newsome SD. Early aggressive treatment approaches for multiple sclerosis. Curr Treat Options Neurol 2021;23:19.
8. Ziemssen T, Derfuss T, de Stefano N et al. Optimizing treatment success in multiple sclerosis. J Neurol 2016;263:1053-65.
9. Giovannoni G, Butzkueven H, Dhib-Jalbut S et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord 2016;9 Suppl 1:S5-S48.
10. Hobart J, Bowen A, Pepper G et al. International consensus on quality standards for brain health-focused care in multiple sclerosis. Mult Scler 2019;25:1809-18.
11. Luna G, Alping P, Burman J et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol 2020;77:184-91.
12. ClinicalTrials.gov. Determining the Effectiveness of earLy Intensive Versus Escalation Approaches for RRMS (DELIVER-MS). 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT03535298 (Accessed April 2022).
13. ClinicalTrials.gov. Traditional versus Early Aggressive Therapy for Multiple Sclerosis Trial (TREAT-MS). 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03500328 (Accessed April 2022).
14. Coyle PK. Commentary: The multiple sclerosis controversy: is it escalation or induction high efficacy? Neurotherapeutics 2020;17:971-2.
15. Ontaneda D, Tallantyre E, Kalincik T et al. Early highly effective versus escalation treatment approaches in relapsing multiple sclerosis. Lancet Neurol 2019;18:973-80.
